Does DeSci need Pump? From the dilemma of the pharmaceutical industry

Reprinted from panewslab
02/11/2025·29D
Original text: Decentralised.co
Compiled by: Yuliya, PANews
Science has always been the biggest catalyst for human progress. However, when the word "science" is mentioned today, people often respond to skepticism. When headlines claim that "science shows...", it is more likely to trigger ridicule than real interest. This growing sense of disillusionment is not without reason – science has increasingly become a marketing term, diluted by corporate interests, deviating from its fundamental purpose of promoting the development of human knowledge and well-being.
Decentralized Science (DeSci), as a new paradigm, promises to rebuild scientific research on a stronger basis. Currently, the DeSci project focuses on the pharmaceutical field, which is one of the most important resource to improve humans - health.
The financial crisis of scientific innovation
The traditional scientific funding system has become dilapidated. Academic researchers spend up to 40% of their time writing grant applications, with success rates below 20%. With the decrease in federal funds, private funds have increased, but are mainly concentrated in the hands of large enterprises.
The pharmaceutical industry has evolved into a high-risk game that is not conducive to innovation. Consider the reality that only 1 out of every 10,000 found compounds can be successfully marketed. This process is extremely cruel. Only 10% of the drugs entering clinical trials are FDA-approved, and the entire process takes up to 15 years, with each successful drug costing more than $2.6 billion.
In the 1990s, the concentration of the pharmaceutical industry seemed to be a boon—it brought efficiency, streamlined supply chains, and allowed drug discovery to expand rapidly. However, this precision machine, initially as an innovation engine, has evolved into a bottleneck, with the same players hindering progress in order to maintain their monopoly position, resulting in a surge in costs.
In the current model, a biotech startup will spend years seeking NIH’s funding for early detection and then raise $15 million in Series A financing to enter preclinical trials. If successful, it licenses intellectual property to large pharmaceutical companies, which invests more than $1 billion in clinical trials and commercialization.
This is where the incentive mechanism is distorted. Rather than focusing on groundbreaking new therapies, big pharma companies have mastered a more profitable game: patent manipulation. The strategy is simple: when a lucrative drug patent is about to expire, dozens of secondary patents are applied for minor modifications—new dosing methods, slightly changed formulas, or even just new uses of the same drug. .
Take AbbVie’s anti-inflammatory drug, Summela, as an example. For many years, Summela has been one of the world's best-selling drugs, with annual revenue of over $20 billion. Its original patent expired in 2016, but AbbVie filed more than 100 additional patents to prevent competition from generic drugs. This legal action delays the entry of affordable alternatives into the market, leaving patients and healthcare systems with billions of dollars.
In the recent DeSci debate between Tarun Chitra and benjels , the question of stagnation of pharmaceutical innovation has been raised and the Eroom law (the opposite of Moore's law) is cited.
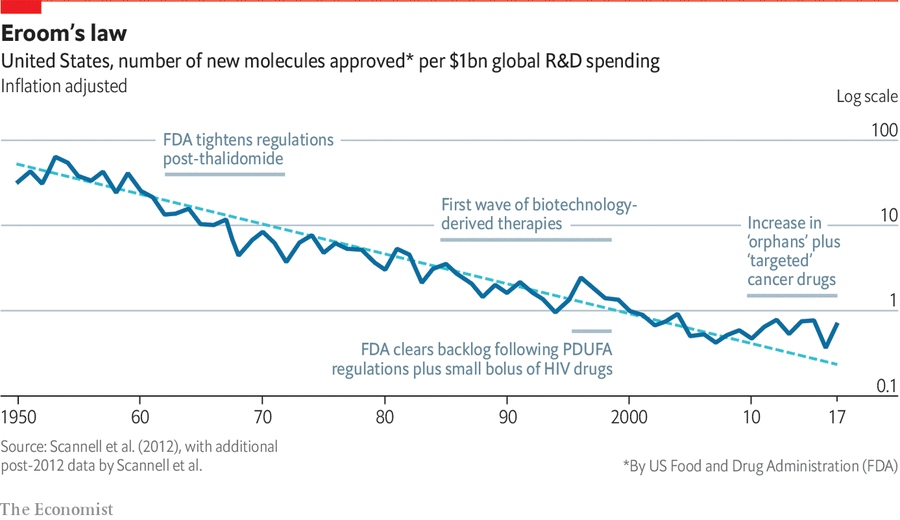
These practices reflect a bigger problem: innovation is captured by profit motives. Pharmaceutical companies invest resources in fine-tuning of existing drugs—making minor chemical modifications or finding new drug delivery mechanisms—not because it can bring significant health benefits, but because new patents can be obtained and profitable periods can be extended.
Science on a better track
Meanwhile, the global research community full of talent and creativity is still excluded from this process. Young researchers are limited by limited funding, red tape, and a "publishing or extinction" culture that values sensational rather than meaningful research. The results resulted in a severe lack of funding for rare diseases, neglected tropical diseases and early exploratory research.
DeSci is essentially a coordination mechanism. It brings together human capital around the world - biologists, chemists, researchers, and researchers, allowing them to synthesize, test and iterate without relying on traditional institutions. The funding model has also been reimagined. Decentralized autonomous organizations (DAOs) and tokenized incentives replace government funding or corporate sponsorship, making capital acquisition more democratic.
The traditional pharmaceutical supply chain is a rigid and isolated process dominated by a few gatekeepers. It usually follows a linear path: centralized data generation, closed laboratory discovery, high-cost trials, exclusive manufacturing and limited allocation. Each link is optimized for profit rather than accessibility or collaboration.
By contrast, DeSci introduces a chain of open collaboration, reimagining each stage, making participation more democratizing and accelerating innovation. It is mainly reflected in the following aspects:
1. Data and Infrastructure
Traditional model: data is proprietary, decentralized, and often inaccessible. Research institutions and pharmaceutical companies hoard data sets to maintain a competitive advantage.
DeSci model: The platform brings together and democratizes access to scientific data to create a foundation for transparent collaboration.
Example: yesnoerror uses AI to check mathematical errors in published papers to improve the reproducibility and credibility of the study.
2. Discovery and research
Traditional model: Discovery occurs in closed academic or corporate laboratories, subject to funding priorities and intellectual property issues.
DeSci Model: DAO directly fundes early research, enabling scientists to explore breakthrough ideas without institutional red tape.
Example: VitaDAO has raised millions of dollars to fund longevity research to support cellular aging and drug discovery projects that were otherwise difficult to obtain. HairDAO is a collective of researchers and patients, recording experiences with different compounds in treating hair loss.
3. Market
Traditional mode: controlled by an intermediary. Researchers rely on traditional publishers, conferences and networks to share discovery and access resources.
DeSci Model: Decentralized markets connect researchers with funders and tools globally.
Example: Bio Protocol provides researchers with a platform to create BioDAOs—these DAOs are dedicated to researching new compounds, providing ongoing funding for newly generated biotech assets, and providing a liquidity market for tokenized IPs. Compared to the AI proxy field, Bio can be regarded as Virtuals in the DeSci world.
Big Pharmai, as a counterpart of ai16z, launched investment in DeSci tokens on Daos.fun. Their AUM has exceeded $1 million and plans to launch its own Bio proxy framework.
4. Experiment and verification
Traditional model: Preclinical and clinical trials are expensive, usually limited to large pharmaceutical companies. Transparency is minimized, failures are often hidden.
DeSci model: platform decentralized experiment, achieving global participation and financial support through tokens.
Example: Pump Science uses binding curves to crowdfund longevity experiments to advance compounds from worm tests to fruit fly to rat tests, and ultimately commercialize them.
Medical researchers can submit drug research proposals on Pump Science , a platform that helps test these drugs on worms and transmit experimental results to the platform front end in real time. Users can speculate on tokens representing these drugs. Two popular tokens, Rif (rifampin) and URO (Urolidin A), are being tested on worms, and if found to extend lifespan, these compounds will enter the commercialization phase and holders will share profits.
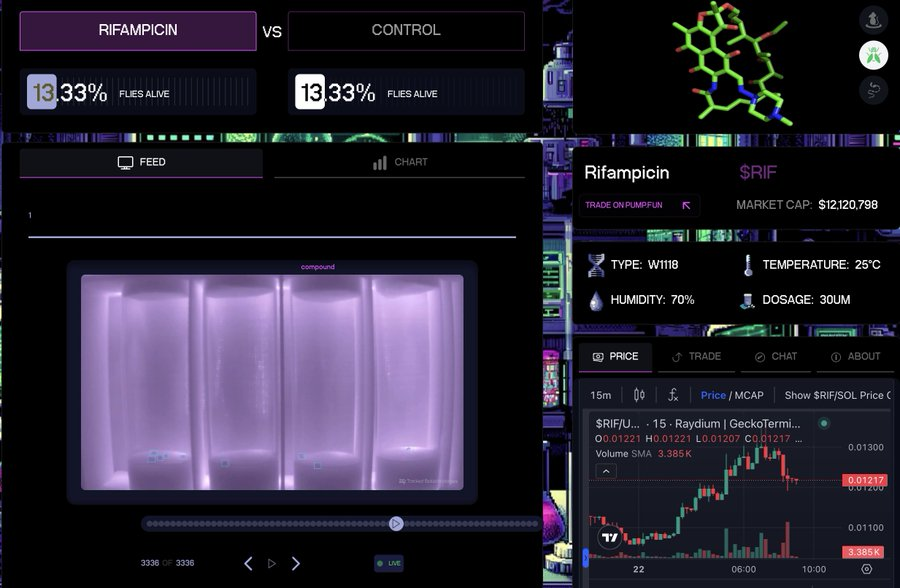
Results of these trials were recorded and broadcast live on Pump.science
5. IP and monetization
Traditional model: Intellectual property is locked in patent monopoly, causing innovation obstacles and drug prices to expand. Patenting a new compound is expensive and painful and complex.
DeSci model: The protocol tokenizes IP, allowing researchers to transparently share and monetize discoveries.
- Example: Molecule 's IP framework enables researchers to fund projects by splitting IP rights into NFTs and tokens, coordinating incentives between scientists and funders. However, this model is still in its early stages. Only a few researchers have experimented with tokenizing their IPs, and when IPs are commercialized, it is still difficult to estimate how profits flow to holders. Furthermore, to ensure that IP is fully protected, researchers may still need to register with traditional government agencies.
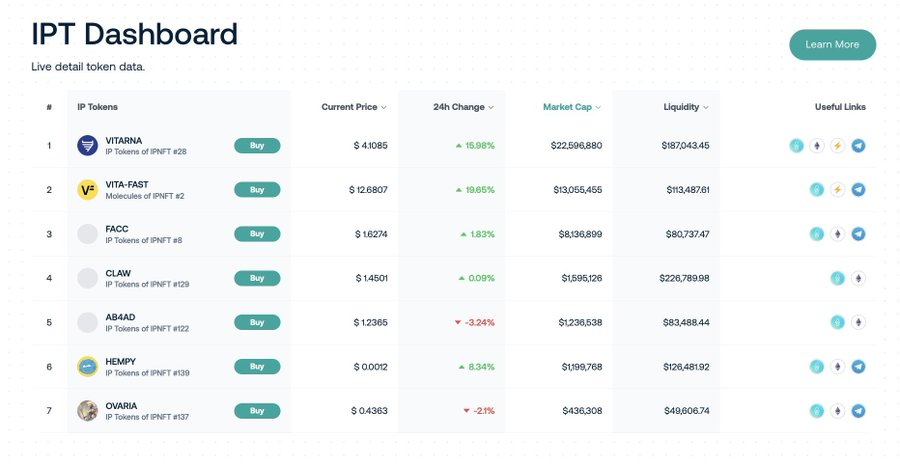
BioDAO has held over $33 million in tokenized IPT through Molecule framework
Accountability Challenge
DAOs face difficulties in coordinating complex tasks and maintaining accountability – few DAOs have demonstrated sustainable success in managing long-term projects. DeSci faces a bigger challenge: it requires researchers to coordinate the handling of complex problems, meet research deadline requirements, and maintain scientific rigor without traditional institutional supervision.
Although traditional science has flaws, it has established peer review and quality control mechanisms. DeSci must either improve these systems or develop a completely new accountability framework. This challenge is particularly severe, given the high risks involved in medical research. A failed NFT project can lose money, but an improperly executed medical trial can be life-threatening.
Critics believe that DeSci is just a speculative act—it 's nothing more than a trading game. This statement is not completely wrong. History shows that new technologies often experience periods of struggle before they achieve breakthrough success and attract public imagination. Just as AI agents gain mainstream attention through projects like aixbt , DeSci may also need a decisive moment to change people’s perceptions.
The future may not be fully developed in the direction envisioned by DeSci supporters. Perhaps it is not about completely replacing traditional institutions, but about creating parallel systems that drive innovation through competition. Or, perhaps, it is to find specific niches—such as rare disease research—those areas where traditional models have failed.
Imagine a world with a brilliant mind that is not bounded by borders or budgets, committed to solving the greatest medical challenge of mankind – a breakthrough in Chinese labs can be instantly verified in Singapore and scaled up in São Paulo.
The pioneers are building this future step by step. Take Bryan Johnson as an example – the independent biohacker is experimenting with hyper-instruction medications and non-traditional therapies. While his approach may be concerned by traditionalists, he represents the spirit of DeSci: experiments take precedence over gatekeeping.



 chaincatcher
chaincatcher